ELECTRONIC INFORMED CONSENT
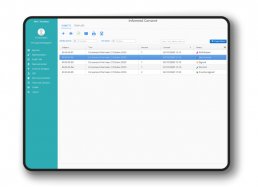
DataRiver has developed the regulatory-compliant and patient-friendly “My Health eConsent”tool, for the management of informed consents in clinical trials on drugs and medical devices, compatible with all eCRF platforms.
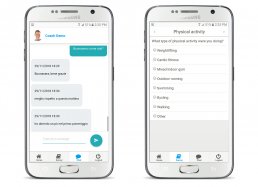
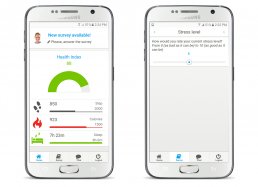
The “My Health eConsent” Tool can be manage by medical staff and patient via PC, tablet and smartphone.
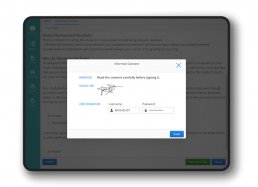
The eConsent module allows to send remotely, illustrate and signing informed consents, ensuring a better patient comprehension and enable them to make conscious decisions regarding the participation in the clinical trial through the use of interactive multimedia components and obtain an higher retention rates.
The use of the eConsent tool allows to improve quality and efficiency of clinical trials, enhancing consent tracking management, and easing the administrative procedures for clinical centers and study teams.
My Health eConsent is GDPR compliant and validated (Computer System Validation) according to international standards and guidelines for clinical trials: ICH GCP, 21 CFR part 11.
RELATED SOLUTIONS
Solutions
RELATED CASES
Case Studies